Characteristic DNA methylation profiles of chorionic villi in recurrent miscarriage
Key Points
- Recurrent miscarriage (RM), the frequency of which is approximately 0.7% of all pregnancies, is defined as three or more consecutive miscarriages before week 22 of gestation. Although RM is one of the most distressing complications of pregnancy, causes of RM remain unknown in a quarter of cases; the frequency of unexplained RM with a normal embryonic karyotype was 5-25% according to recent microarray analysis.
- We examined genome-wide DNA methylation in chorionic villi and decidual tissues from patients suffering RM and from healthy women who had undergone artificial abortion (AA) (n=5 each), and DNA methylation profiles of chorionic villi, but not decidua, in RM patients were clearly distinct from controls.
- Among the differentially methylated genes, the enhancer region of SPATS2L was significantly more highly methylated in RM patients (n=19) than in AA controls (n=19; mean methylation level, 52.0 %-vs.-28.9%, P<0.001), resulting in reduced expression of SPATS2L protein in the former. Functionally, depletion of SPATS2L in extravillous trophoblast cells decreased their invasion and migration abilities.
- Our data indicate that particularly the chorionic villi in RM patients exhibit distinct DNA methylation profiles compared with normal pregnancies and that this changed DNA methylation status may impede the progression of embryo development via the altered expression of genes such as SPATS2L in the villi.
Summary
Dysregulation of transcriptional programs that are tightly regulated by DNA methylation during placental and fetal development at different gestational stages, may cause recurrent miscarriage. Here, we examined genome-wide DNA methylation in chorionic villi and decidual tissues from patients suffering RM and from healthy women who had undergone artificial abortion (n= 5 each). We found that 13,426 and 5,816 CpG sites were differentially methylated in chorionic villi and decidua, respectively. DNA methylation profiles of chorionic villi, but not decidua, in RM patients was clearly distinct from AA controls. Among the differentially methylated genes, the enhancer region of SPATS2L was significantly more highly methylated in RM patients (n= 19) than AA controls (n= 19; mean methylation level, 52.0%-vs.-28.9%, P< 0.001), resulting in reduced expression of SPATS2L protein in the former. Functionally, depletion of SPATS2L in extravillous trophoblast cells decreased their invasion and migration abilities.
Our data indicate that particularly the chorionic villi in RM patients exhibit distinct DNA methylation profiles compared with normal pregnancies and that this changed DNA methylation status may impede the progression of embryo development via the altered expression of genes such as SPATS2L in the villi.
Dysregulation of transcriptional programs that are tightly regulated by DNA methylation during placental and fetal development at different gestational stages, may cause recurrent miscarriage. Here, we examined genome-wide DNA methylation in chorionic villi and decidual tissues from patients suffering RM and from healthy women who had undergone artificial abortion (n= 5 each). We found that 13,426 and 5,816 CpG sites were differentially methylated in chorionic villi and decidua, respectively. DNA methylation profiles of chorionic villi, but not decidua, in RM patients was clearly distinct from AA controls. Among the differentially methylated genes, the enhancer region of SPATS2L was significantly more highly methylated in RM patients (n= 19) than AA controls (n= 19; mean methylation level, 52.0%-vs.-28.9%, P< 0.001), resulting in reduced expression of SPATS2L protein in the former. Functionally, depletion of SPATS2L in extravillous trophoblast cells decreased their invasion and migration abilities.
Our data indicate that particularly the chorionic villi in RM patients exhibit distinct DNA methylation profiles compared with normal pregnancies and that this changed DNA methylation status may impede the progression of embryo development via the altered expression of genes such as SPATS2L in the villi.
Research Background
Recurrent miscarriage (RM), the frequency of which is approximately 0.7% of all pregnancies, is defined as three or more consecutive miscarriages before week 22 of gestation, and RM is one of the most distressing complications of pregnancy. However, causes of RM remain unknown in a quarter of cases; the frequency of unexplained RM with a normal embryonic karyotype was 5-25% according to a recent microarray analysis because the prognosis of RM patients who had previously had a normal embryonic karyotype is poorer than that of patients with previous embryonic aneuploidy, elucidating the causes of RM is a matter of immediate concern.
DNA methylation in gene regulatory regions, such as enhancers and promoters, is closely associated with gene expression, including of lineage-specific genes. During early embryogenesis after fertilization, dynamic epigenetic reprogramming ensures the correct development of the embryos. Recent studies documented altered DNA methylation in certain sets of genes, such as imprinting loci, in the chorionic villi of RM, or sporadic miscarriage after in vitro fertilization. In the current study, we investigated genome-wide DNA methylation profiles in both chorionic villi and decidual tissues from the products of conception (POC) in patients with euploid RM were detected, compared with the same tissues from women with artificial abortions as controls of the same gestational week.
Recurrent miscarriage (RM), the frequency of which is approximately 0.7% of all pregnancies, is defined as three or more consecutive miscarriages before week 22 of gestation, and RM is one of the most distressing complications of pregnancy. However, causes of RM remain unknown in a quarter of cases; the frequency of unexplained RM with a normal embryonic karyotype was 5-25% according to a recent microarray analysis because the prognosis of RM patients who had previously had a normal embryonic karyotype is poorer than that of patients with previous embryonic aneuploidy, elucidating the causes of RM is a matter of immediate concern.
DNA methylation in gene regulatory regions, such as enhancers and promoters, is closely associated with gene expression, including of lineage-specific genes. During early embryogenesis after fertilization, dynamic epigenetic reprogramming ensures the correct development of the embryos. Recent studies documented altered DNA methylation in certain sets of genes, such as imprinting loci, in the chorionic villi of RM, or sporadic miscarriage after in vitro fertilization. In the current study, we investigated genome-wide DNA methylation profiles in both chorionic villi and decidual tissues from the products of conception (POC) in patients with euploid RM were detected, compared with the same tissues from women with artificial abortions as controls of the same gestational week.
Research Results
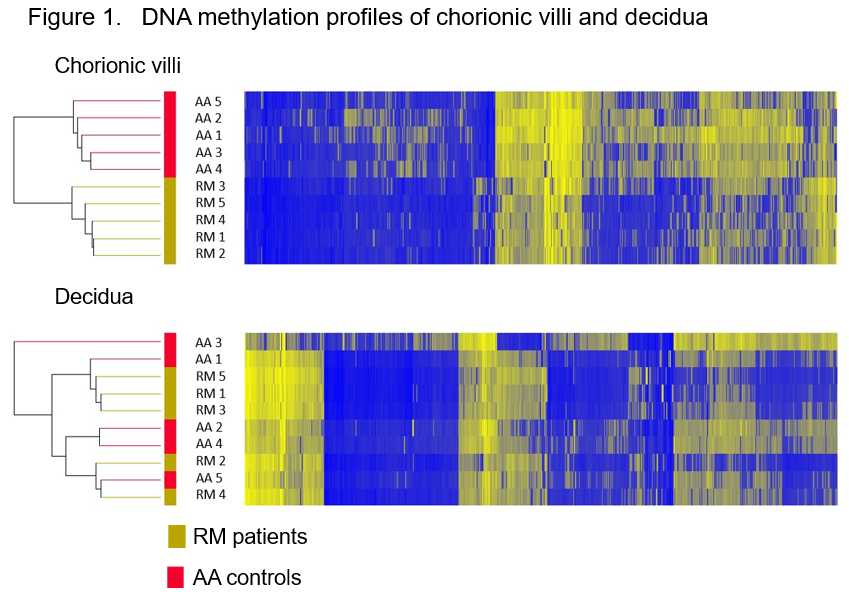
First, genome-wide DNA methylation profiles were established using POC tissues from RM patients (n=5) and AA controls (n=5) whose karyotypes were diagnosed as normal. Unsupervised two-way hierarchical cluster analysis using all differentially methylated probes (DMPs) showed clearly distinct DNA methylation signatures between RM and AA in chorionic villi but not in decidua (Figure 1).
First, genome-wide DNA methylation profiles were established using POC tissues from RM patients (n=5) and AA controls (n=5) whose karyotypes were diagnosed as normal. Unsupervised two-way hierarchical cluster analysis using all differentially methylated probes (DMPs) showed clearly distinct DNA methylation signatures between RM and AA in chorionic villi but not in decidua (Figure 1).
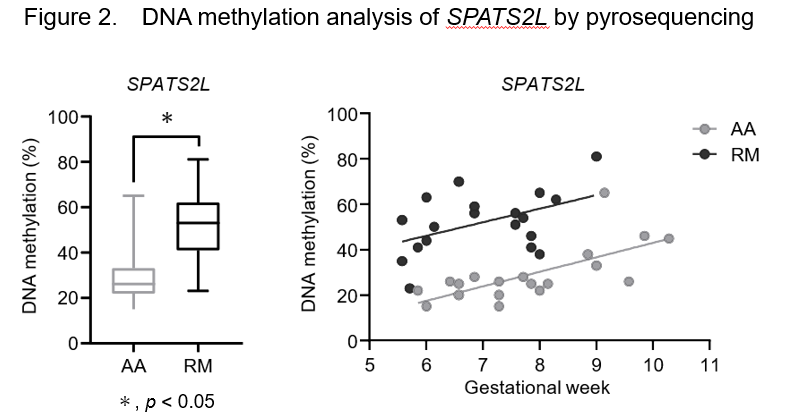
Next, We analyzed DMPs in the enhancer regions to identify those genes that were consistently methylated in the chorionic villi of RM patients. We designed 5 quantitative pyrosequencing DNA methylation assays for differently methylated probe regions. Of the five genes, DNA methylation levels of SPATS2L, MAST4, and EXOC6B were significantly higher in chorionic villi from RM patients (n=19) than AA controls (n=19, P<0.01) Interestingly, there was a clear positive correlation between DNA methylation levels and gestational weeks in SPATS2L in both RM and AA groups (R2=0.217 and R2=0.485 in RM and AA, respectively) (Figure 2).
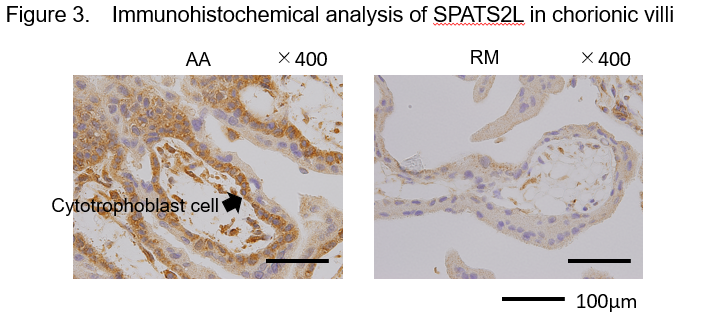
Because a significantly higher level of DNA methylation was detected in the enhancer region of SPATS2L in chorionic villi of RM patients than in AA controls, we quantified the level of SPATS2L protein therein. We found that this protein is expressed mainly in the cytoplasm of cytotrophoblast cells but at a lower level in RM patients than in AA controls (Figure 3).
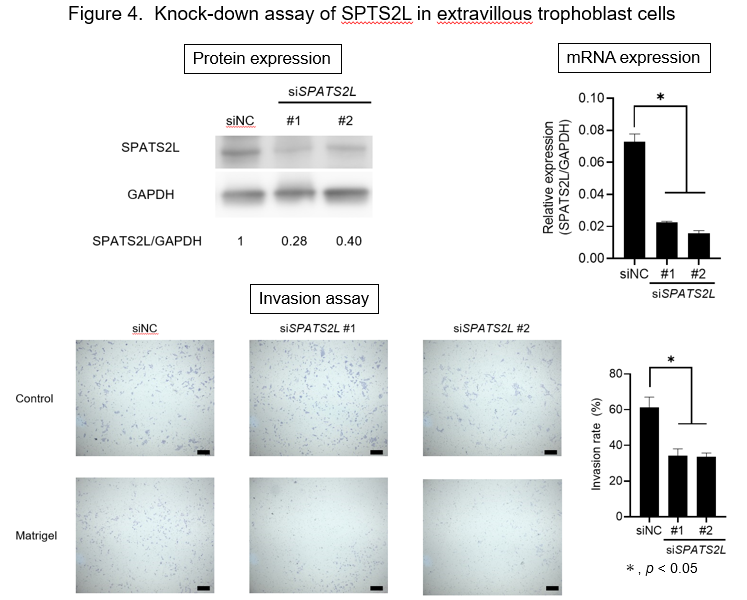
To assess the function of SPATS2L in the trophoblast cells, we knocked down SPATS2L gene expression by two independent siRNAs in a human extravillous trophoblast cell line, HTR8/SVneo. The depletion of SPATS2L significantly reduced the invasive ability of these cells (P< 0.05) (Figure 4). Furthermore, the scratch assay revealed significantly decreased cell migration of SPATS2L-depleted cells (P< 0.05). Taken together, these data imply that altered SPATS2L expression may affect cell migration and invasion during the early development of the placenta.
To assess the function of SPATS2L in the trophoblast cells, we knocked down SPATS2L gene expression by two independent siRNAs in a human extravillous trophoblast cell line, HTR8/SVneo. The depletion of SPATS2L significantly reduced the invasive ability of these cells (P< 0.05) (Figure 4). Furthermore, the scratch assay revealed significantly decreased cell migration of SPATS2L-depleted cells (P< 0.05). Taken together, these data imply that altered SPATS2L expression may affect cell migration and invasion during the early development of the placenta.
Research Summary and Future Perspective
Our genome-wide DNA methylation analysis that simultaneously examined the chorionic villi and decidual tissues derived from the same RM patients revealed clearly distinct disease-associated DNA methylation profiles in chorionic villi, but not in maternal decidua. This indicates the altered DNA methylation program in a particular tissue is involved in the maintenance of a successful pregnancy. Furthermore, dysregulation of an affected gene, SPATS2L, downregulated the function of extravillous trophoblast cells. Such epigenetic alterations may be induced in response to environmental factors on the background of certain genetic polymorphisms. Further studies with a larger, well-characterized population will allow us to better understand the epigenome-associated mechanism responsible for RM.
Our genome-wide DNA methylation analysis that simultaneously examined the chorionic villi and decidual tissues derived from the same RM patients revealed clearly distinct disease-associated DNA methylation profiles in chorionic villi, but not in maternal decidua. This indicates the altered DNA methylation program in a particular tissue is involved in the maintenance of a successful pregnancy. Furthermore, dysregulation of an affected gene, SPATS2L, downregulated the function of extravillous trophoblast cells. Such epigenetic alterations may be induced in response to environmental factors on the background of certain genetic polymorphisms. Further studies with a larger, well-characterized population will allow us to better understand the epigenome-associated mechanism responsible for RM.
Publication
Scientific Reports
Title: Characteristic DNA methylation profiles of chorionic villi in recurrent miscarriage
Publication date: 10 am UK by default on 27th July 2022
Author:
Yosuke Matsumoto1, Keiko Shinjo2, Shoko Mase1, Masaki Fukuyo3, Kosuke Aoki4,
Fumiko Ozawa1, Hiroyuki Yoshihara1, Shinobu Goto1, Tamao Kitaori1, Yasuhiko Ozaki1,
Satoru Takahashi5, Atsushi Kaneda3, Mayumi Sugiura‑Ogasawara1 & Yutaka Kondo2
Affiliation:
1. Department of Obstetrics and Gynecology, Nagoya City University Graduate School of Medical Sciences, Nagoya 467-8601, Japan.
2. Division of Cancer Biology, Nagoya University Graduate School of Medicine, Nagoya 466-8550, Japan.
3. Department of Molecular Oncology, Graduate School of Medicine, Chiba University,
Chiba 260-8670, Japan.
4. Department of Neurosurgery, Nagoya University Graduate School of Medicine, Nagoya 466-8550, Japan.
5. Department of Experimental Pathology and Tumor Biology, Nagoya City University
Graduate School of Medical Sciences, Nagoya 467-8601, Japan.
DOI: 10.1038/s41598-022-15656-y
Scientific Reports
Title: Characteristic DNA methylation profiles of chorionic villi in recurrent miscarriage
Publication date: 10 am UK by default on 27th July 2022
Author:
Yosuke Matsumoto1, Keiko Shinjo2, Shoko Mase1, Masaki Fukuyo3, Kosuke Aoki4,
Fumiko Ozawa1, Hiroyuki Yoshihara1, Shinobu Goto1, Tamao Kitaori1, Yasuhiko Ozaki1,
Satoru Takahashi5, Atsushi Kaneda3, Mayumi Sugiura‑Ogasawara1 & Yutaka Kondo2
Affiliation:
1. Department of Obstetrics and Gynecology, Nagoya City University Graduate School of Medical Sciences, Nagoya 467-8601, Japan.
2. Division of Cancer Biology, Nagoya University Graduate School of Medicine, Nagoya 466-8550, Japan.
3. Department of Molecular Oncology, Graduate School of Medicine, Chiba University,
Chiba 260-8670, Japan.
4. Department of Neurosurgery, Nagoya University Graduate School of Medicine, Nagoya 466-8550, Japan.
5. Department of Experimental Pathology and Tumor Biology, Nagoya City University
Graduate School of Medical Sciences, Nagoya 467-8601, Japan.
DOI: 10.1038/s41598-022-15656-y


