Passport Control for Glycan Maturation
Passport Control for Glycan Maturation:Discovery of a Molecular Tag Enhancing Biopharmaceutical Quality
Summary Text
A new technology enhances critical modifications of glycoprotein glycans, specifically galactosylation and sialylation, by adding a “passport sequence” that promotes efficient transport through the secretory pathway. This approach improves the quality and efficiency of therapeutic glycoproteins, advancing the development of high-quality biopharmaceuticals.
Full Text
A collaborative research group, including researchers from Nagoya City University, National Institutes of Natural Sciences, and RIKEN has uncovered a groundbreaking molecular tool, the "passport sequence," that significantly improves the production efficiency and quality of glycoproteins, such as blood coagulation factor VIII and erythropoietin (EPO). This discovery holds great promise for the development of high-quality biopharmaceuticals.
The study focuses on a short, 10-amino-acid sequence called the "passport sequence," which facilitates the efficient trafficking of glycoproteins through the secretory pathway. When attached to target proteins, this sequence enhances their interactions with glycosylation enzymes in the Golgi apparatus, leading to significantly increased galactosylation and sialylation—critical modifications that impact protein stability and efficacy.
The researchers revealed that the passport sequence selectively interacts with a Golgi protein called NUCB1, which in turn promotes the function of the glycosyltransferase B4GALT1. This mechanism not only improves glycan maturation but also provides a novel strategy for controlling glycosylation in therapeutic glycoproteins, ultimately improving their pharmacokinetics and therapeutic properties.
The researchers emphasize "This discovery not only offers a new approach to enhancing the production and quality of biopharmaceutical glycoproteins but also provides deeper insights into the complex mechanisms underlying protein glycosylation,"
A new technology enhances critical modifications of glycoprotein glycans, specifically galactosylation and sialylation, by adding a “passport sequence” that promotes efficient transport through the secretory pathway. This approach improves the quality and efficiency of therapeutic glycoproteins, advancing the development of high-quality biopharmaceuticals.
Full Text
A collaborative research group, including researchers from Nagoya City University, National Institutes of Natural Sciences, and RIKEN has uncovered a groundbreaking molecular tool, the "passport sequence," that significantly improves the production efficiency and quality of glycoproteins, such as blood coagulation factor VIII and erythropoietin (EPO). This discovery holds great promise for the development of high-quality biopharmaceuticals.
The study focuses on a short, 10-amino-acid sequence called the "passport sequence," which facilitates the efficient trafficking of glycoproteins through the secretory pathway. When attached to target proteins, this sequence enhances their interactions with glycosylation enzymes in the Golgi apparatus, leading to significantly increased galactosylation and sialylation—critical modifications that impact protein stability and efficacy.
The researchers revealed that the passport sequence selectively interacts with a Golgi protein called NUCB1, which in turn promotes the function of the glycosyltransferase B4GALT1. This mechanism not only improves glycan maturation but also provides a novel strategy for controlling glycosylation in therapeutic glycoproteins, ultimately improving their pharmacokinetics and therapeutic properties.
The researchers emphasize "This discovery not only offers a new approach to enhancing the production and quality of biopharmaceutical glycoproteins but also provides deeper insights into the complex mechanisms underlying protein glycosylation,"
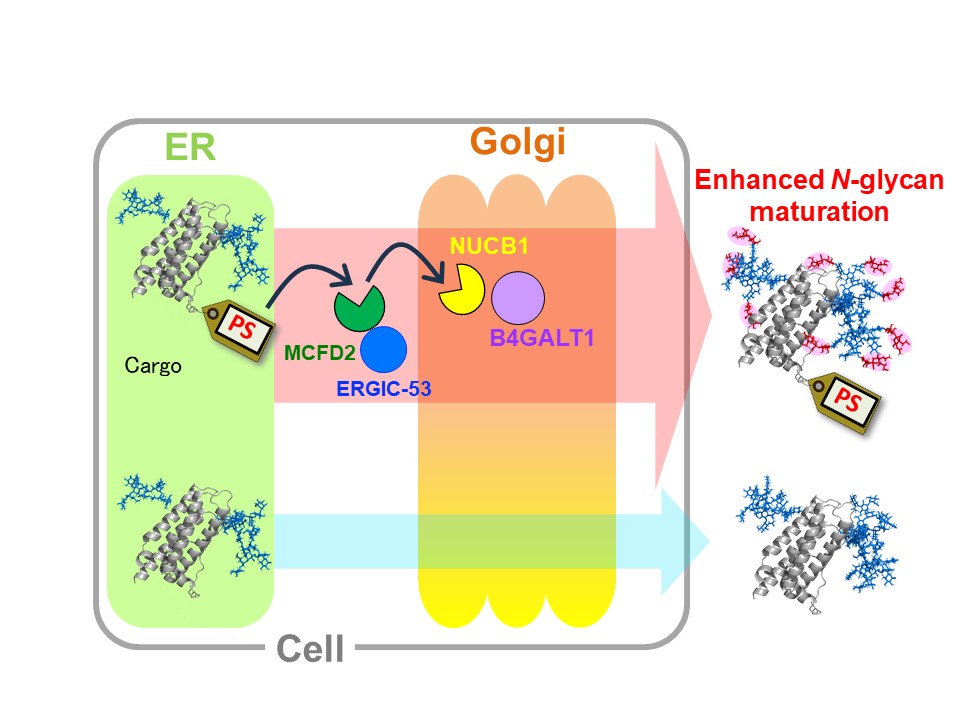
Passport sequence tag promotes glycan maturation
Glycoproteins tagged with the passport sequence (PS) are recognized by cargo transport receptors and transferred to NUCB1. Furthermore, galactosylation and subsequent sialylation of the glycoproteins are promoted by galactosyltransferases located in proximity to NUCB1.
Reference
Journal:iScience
DOI:10.1016/j.isci.2024.111457
Author
Hirokazu Yagi and Koichi Kato
Journal:iScience
DOI:10.1016/j.isci.2024.111457
Author
Hirokazu Yagi and Koichi Kato


