Molecular and Cellular Pharmacology
Staffs
Hisao Yamamura, Ph.D., Professor
Yoshiaki Suzuki, Ph.D., Assistant Professor
Rubii Kondo, Ph.D., Assistant Professor
Yoshiaki Suzuki, Ph.D., Assistant Professor
Rubii Kondo, Ph.D., Assistant Professor
Research Project
- Molecular Functions of Ion Channels and Related Diseases / Channelopathy
- Live imaging of Cellular Ion Dynamics and Molecular Complexes Containing Ion Channels
- Discovery of Drugs acting on Ion Channels and Screening System
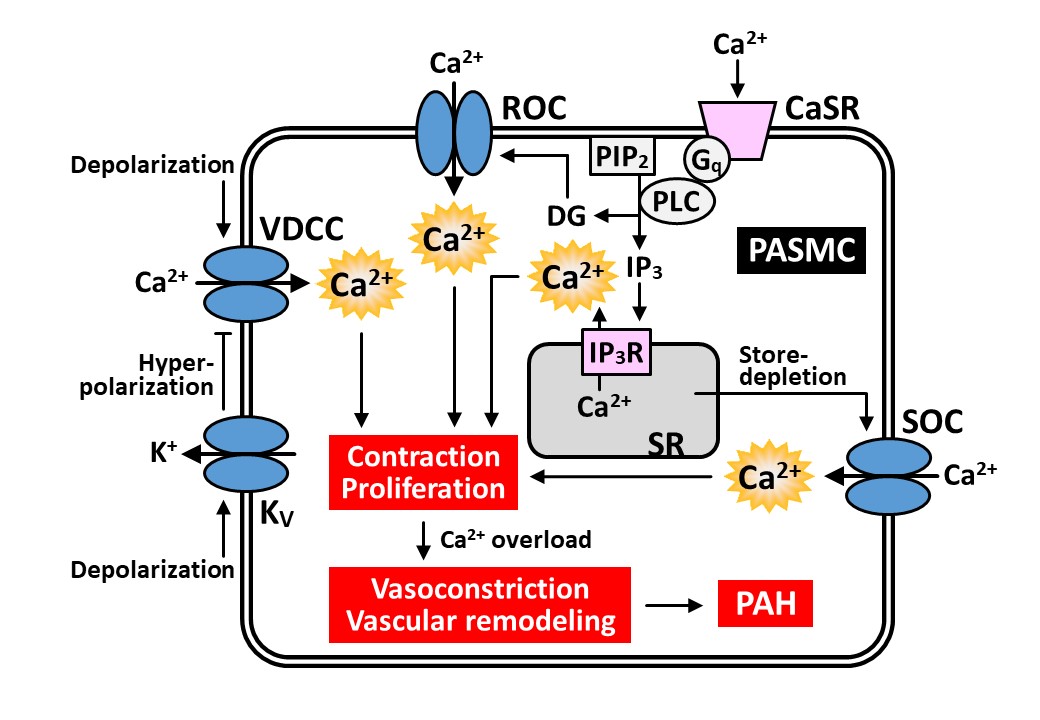
1) Ca2+ signaling and ion channels in pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is classified as group 1 of pulmonary hypertension. PAH is a progressive and fatal disease of the pulmonary artery. The major pathogenesis of PAH is sustained vasoconstriction and vascular remodeling of the pulmonary artery. These pathogeneses cause progressive elevations in pulmonary vascular resistance and pulmonary arterial pressure (PAP) in PAH patients. Elevated PAP leads to right heart failure and finally death. A central aspect of pulmonary vascular remodeling is medial hypertrophy, which is caused by the enhanced proliferation and reduced apoptosis of pulmonary arterial smooth muscle cells (PASMCs). Excitable abnormality in the pulmonary artery of PAH patients are mostly mediated by an elevated cytosolic [Ca2+]. Enhanced Ca2+ signaling have been reported in PASMCs from PAH patients. PASMCs express several Ca2+-permeable channels including voltage-dependent Ca2+ channels, receptor-operated Ca2+ channels, and store-operated Ca2+ channels. The expression levels of these Ca2+ channels are increased in the lung tissues and PASMCs of PAH patients. Targeting these Ca2+ channels in PASMCs may help develop novel therapeutic approach for PAH.
2) Vasculature remodeling by E-T coupling
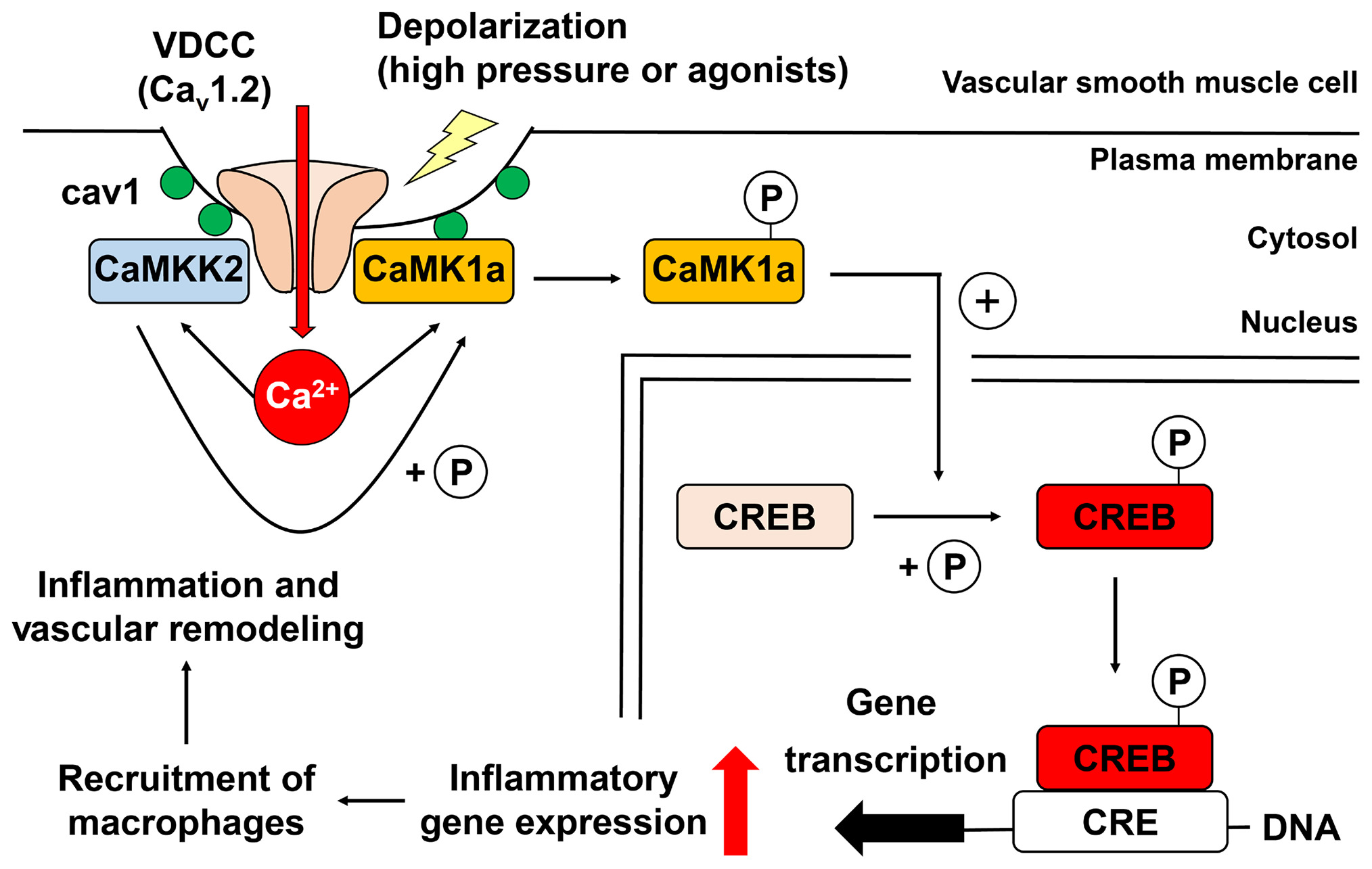
Excitation–transcription (E-T) coupling can initiate and modulate essential physiological or pathological responses in cells, such as neurons and cardiac myocytes. Although vascular myocytes also exhibit E-T coupling in response to membrane depolarization, the underlying molecular mechanisms are unknown. Our study reveals that E-T coupling in vascular myocytes converts intracellular Ca2+ signals into selective gene transcription related to chemotaxis, leukocyte adhesion, and inflammation. Our discovery identifies a mechanism for vascular remodeling as an adaptation to increased circumferential stretch.
3) Ca2+ signaling in hepatic stallate cells
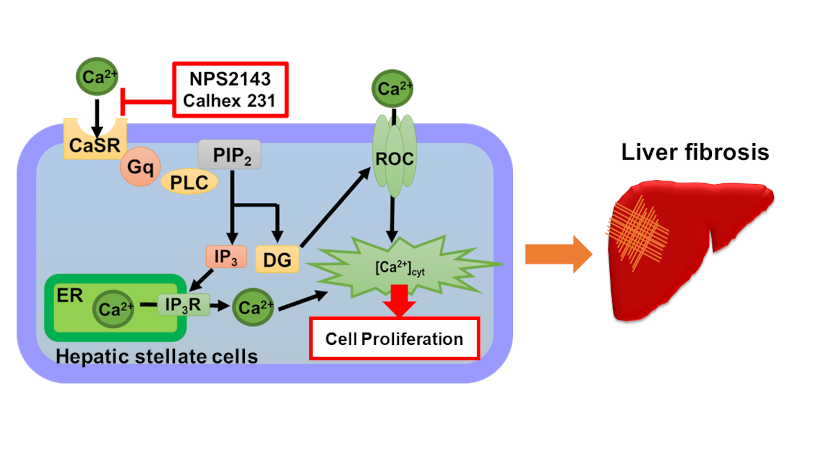
Hepatic stellate cells (HSCs) play a significant role in the development of chronic liver diseases. Hepatic damage activates HSCs and results in hepatic fibrosis. The functions of activated HSCs require an increase in the cytosolic Ca2+ concentration ([Ca2+]cyt). However, the regulatory mechanisms underlying Ca2+ signaling in activated HSCs remain largely unknown. CaSRs are functionally expressed in activated HSCs and regulate Ca2+ signaling and cell proliferation. The present results provide insights into the molecular mechanisms underlying hepatic fibrosis and will contribute to the development of potential therapeutic targets.
Contact Information
Graduate School of Pharmaceutical Sciences, Nagoya City University,
3-1, Tanabe-dori, Mizuho-ku, Nagoya 467-8603, Japan
E-mail:yamamura@phar.nagoya-cu.ac.jp
TEL/FAX:+81-52-836-3431
3-1, Tanabe-dori, Mizuho-ku, Nagoya 467-8603, Japan
E-mail:yamamura@phar.nagoya-cu.ac.jp
TEL/FAX:+81-52-836-3431